Clinical Research Associate Responsibilities
Clinical Research Associate Responsibilities
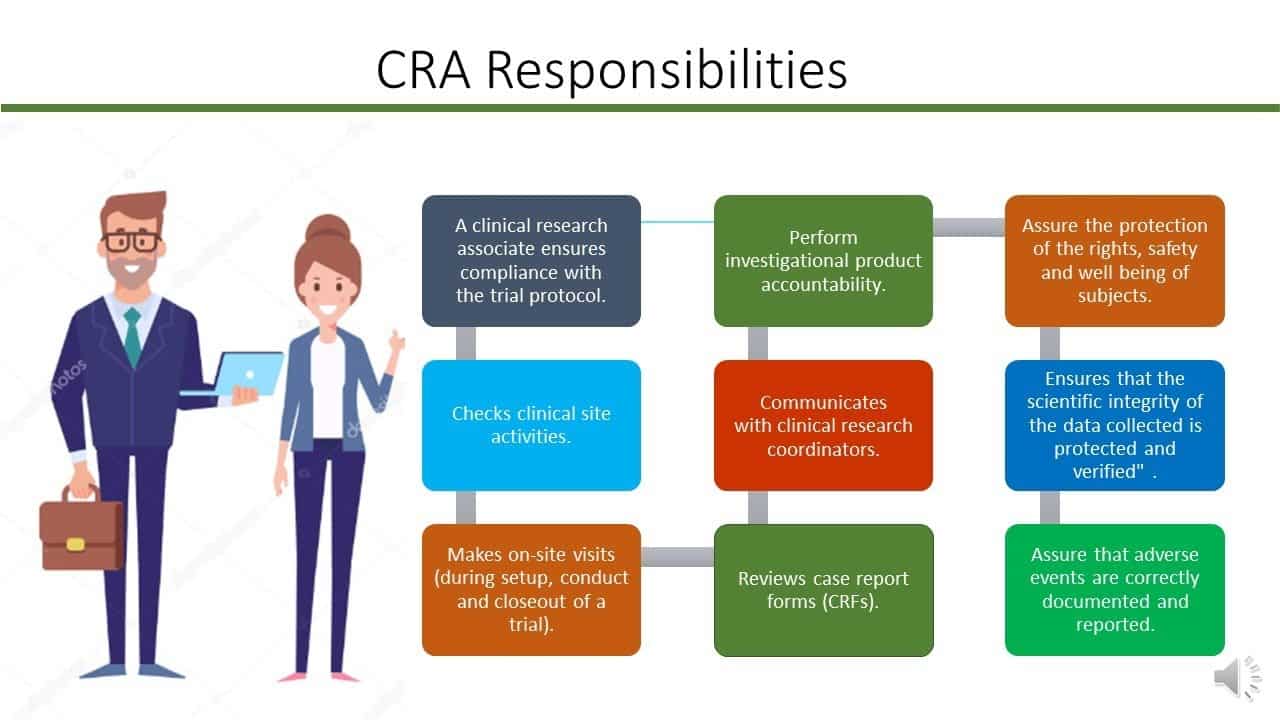
The following article enumerates the various responsibilities of a Clinical Research Associate in the various testing processes in the field of pharmaceuticals. The responsibilities of a Clinical Research Associate spans over a vast range but the primary function is the ability to conduct tests and trials of products on various subjects.
The significant role of a clinical research associate lies in setting up and monitoring such trials.
Clinical Research Associate Job Responsibilities
- Must develop and design suitable methodologies for the trials.
- Should have good communication skills as a Clinical Research Associate is responsible for putting forward the methodologies to a committee.
- Coordinating with doctors and medical professionals on the conducting of the trials.
- Must make sure the trial center is monitored on a regular basis.
- Must maintain a record of all data and records of the trial.
- Must have a good knowledge of existing laws and legal protocols involved in the testing and production of any new drug.
- Must be able to train staff.
- They are the essential communication line between the investigator and sponsor.
- Is liable for the proper conduct and safety throughout trial.
- They need to verify that investigator follow approved protocol & all the GCP procedures.
- Along with this, they must verify the documents or source data & other trial-records whether they are maintained well, complete and accurate.
Category: Pharmaceutical Job Responsibilities